Welcome to InterCS
ISO 13485 Certification
Certification to DIN EN ISO 13485 ensures a high level of quality and regulatory compliance in medical device manufacturing—regardless of company size. Our experts will guide you through the entire certification process, helping you optimize your quality management system and strengthen your market position.


Your advantages at a glance
- Higher productivity and lower risk
- Motivated employees and satisfied customers thanks to efficient processes
- Strengthen your image with ISO 9001 certification
- Proof of legal compliance
- Fulfilment of customer requirements & participation in tenders
- Stand out from the competition
- Possibility of new markets
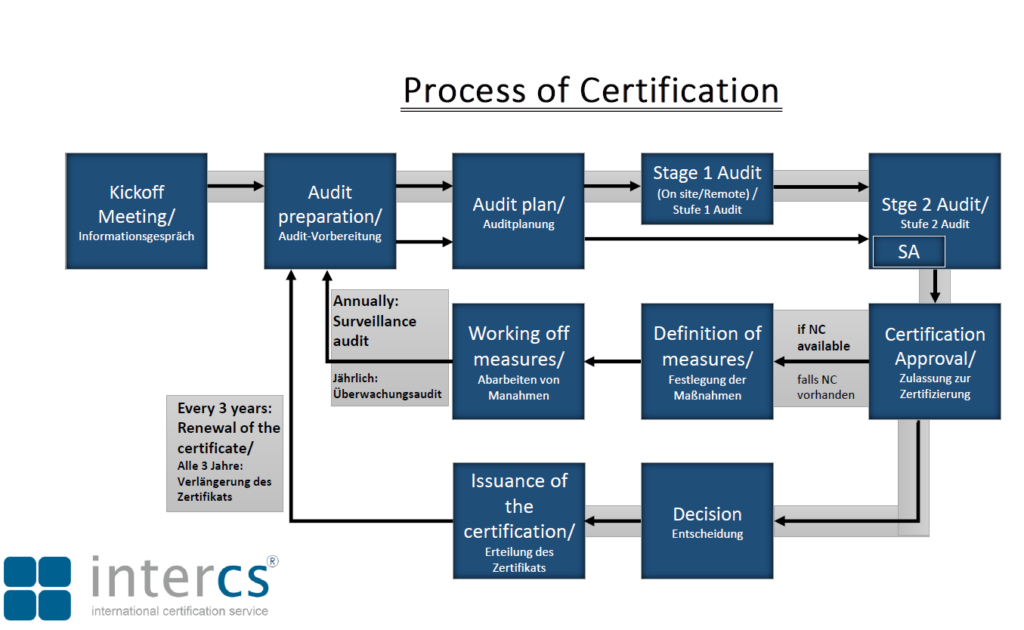
ISO 13485 certification process
Information Meeting and Pre-Audit (Optional)
We start with an informational meeting, either by telephone or in person, to discuss your project and prepare you for ISO 13485 certification. Relevant documents related to your quality management system for medical devices will be reviewed. Optionally, a pre-audit can be performed to assess the current status of your processes.
On-Site or Remote Certification Audit
Our auditors evaluate your company’s readiness for certification. This includes assessing your quality management system, reviewing documented processes, and verifying compliance with medical device regulations. If necessary, corrective actions are reviewed in a follow-up audit.
Audit Report and Evaluation
After the audit, we provide a detailed report assessing the effectiveness of your quality management system and identifying areas for improvement.
Certification
Upon successful completion of the audit, you will receive your ISO 13485 certificate, valid for up to three years.
Surveillance Audits
To ensure ongoing compliance with medical device regulations, we conduct regular surveillance audits throughout the certification period.
Recertification
Before the certificate expires, a recertification audit is conducted to confirm long-term compliance and maintain the benefits of ISO 13485 certification.

Important details about ISO 13485
DIN EN ISO 13485 follows the ‘High Level Structure’ (HLS) and is compatible with other ISO standards. It emphasizes a risk-based approach, ensuring compliance with regulatory requirements and enhancing patient safety through effective quality management.
Why work with us?
Our auditors have many years of experience in cross-industry certifications. With a globally recognised certification, you benefit from maximum value and strengthen your market position in the long term.
Offers within 24 hours
Express certification process within 14 days
Offers for integrated management systems
Contact Us – Get in Touch with Our Experts
Need assistance? Have questions about certification, accreditation, or training? Our expert team is here to help!